Wednesday, November 16, 2011
3 Demonstrations by Miss Leland
Our teacher, Miss Leland performed three demonstrations. One with Ethanol, another with Hydrochloric Acid and Zinc, and the last with baking soda/vinegar. To begin with, she put Ethanol into a soda bottle and swirled it around. It was transforming into a gas. When heat (energy) was added it was supposed to launch forwards, but instead it just stayed lit for a few seconds and went out. Next, she combined baking soda and vinegar. The baking soda and vinegar combined int oCarbon Dioxide. After they were mixed together, she lit two candles. She poured the gas over the candles and they both suddenly went out. This is because fire needs oxygen to continue burning. So, when we poured the Carbon Dioxide over it, it went out because Carbon Dioxide is the opposite of Oxygen. Since the fire was surrounded by CO2 (Carbon Dioxide), it didn't have the Oxygen required for it to maintain the chemical reaction required for the flame to stay consistent. Finally, we did the test with Hydrochloric Acid (HCl) and Zinc (Zn). We created ZnCl2 + H2. We also applied heat part of the way through it. Heat or energy can at times be a catalyst that is needed to start or speed up the chemical reaction. Once the Zinc was introduced, it bubbled and released steam (gas). The gas was Hydrogen. We then added fire and it sped up the process. The Zinc was broken into small pieces by the end of the reaction.
Friday, November 4, 2011
Chemical Reactions and Temperature Lab
In this experiment, we dropped an Alka-Seltzer into different temperatures of water to see what would dissolve faster. We predicted that the hotter the temperature got the faster it would dissolve. The hot water was at 50 degrees Celsius and it took 20 seconds to fully dissolve. The room temperature water was at 25.1 degrees Celsius and took 27 seconds. Finally, the cold water took a minute and 56 seconds to dissolve at 0.8 degrees Celsius.
Alka-Seltzers are made of NaHCO3 and once you combine it with H2O you create CO2. The CO2 are in the bubbles created by the two, Sodium bicarbonate and water, combining. The heat makes the water molecules bounce off each other faster to break the tablet faster.
Wednesday, November 2, 2011
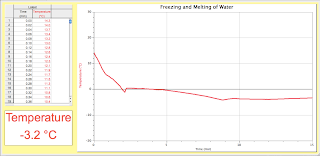
Melting and Freezing of Water Lab
We predicted that the freezing water would be at -2 degrees Celsius and the melting water would be 3.5 degrees Celsius. The actual temperature for the freezing water was -2.364 degrees Celsius. Also, the melting water temperature was 3.268 degrees Celsius.
This is the freezing water's progression.
This is the melting water's progression. The temperature probe immediately froze once we stopped stirring the probe around.
Once, we put it into our warm tap water it completely melted within three minutes. The temperature for the freezing water went down while vise versa for the melting water.
Wednesday, October 19, 2011
Chemistries Greatest Discoveries
There has been amazing advancements in science over the years. To begin, Joseph Priestly discovered oxygen in England. He used mercury as a liquid metal to help discover gases. This man discovered oxygen and helped create the elements we still use today. Next, John Dalton also had a great discovery called The Relative Weights of Ultimate Particles. When it went public he called it Atomic Weights. Also, he developed the Atomic Theory which showed the relationship between atoms and electrons. Thirdly, Joseph Gay-Lussac noticed that gases produced twice the volume he expected. Amedeo Avogadro soon realized that gases had many atoms known as molecules. Also, August Kekule developed a system to help visualize the chemical structure of molecules. He developed a simple yet elegant system. The chemical Benzene had ruined his system. Kekule soon explained it as a ring, solving his problems. Next, Dimitri Mendeleev made a card for each element, for his students, and facts about about each element. This lead him to creating the Periodic Table of Elements. This changed how everyone would learn the elements. In addition, Humphry Davy discovered that electricity reacting with chemicals can transform them. Next, Joseph Thompson used a crooks-tube in the experiments. The stream of electrons bends to magnets. Next, Gilbert Louis explained that electrons and atoms went in shells around the nucleus. A shell can only contain a certain amount of electrons. Two chemical elements might form a compound when they give up or accept electrons from the shells. X-rays were founded in the early 1890's. This radioactivity changes daily life. It powers space crafts, it lets doctors see inside of you, it can cure tumors, it triggers fire alarms, and it lets us tell how old Earth is. Chemists changed the look and feel of the material world. Also, John Hyatt created plastic. Advancement is a good word. A word that means too many things altogether, yet it is what happens every day. Things come and go. These are only a few of what things are to be discovered.
Subscribe to:
Posts (Atom)